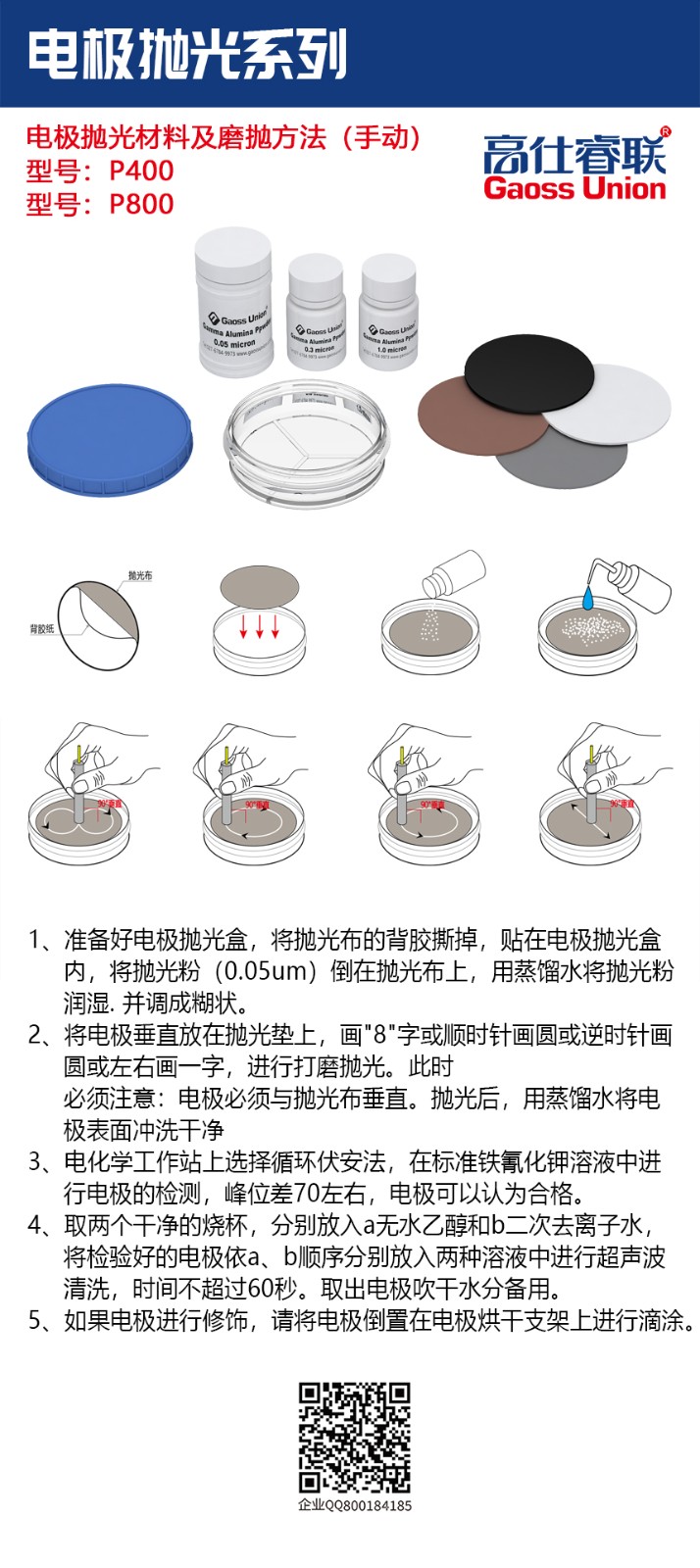
Before using any solid electrode, its surface must be cleaned to remove contamination caused by impurities or impurities adsorbed on the surface. Just as most metal electrode surfaces are prone to the formation of oxide layers, the oxidation of carbon electrode surfaces can generate various oxygen-containing groups (such as alcohols, phenols, carboxyl groups, ketoquinones, and anhydrides), resulting in decreased reproducibility, stability, sensitivity, and loss of selectivity of the electrode.
 WeChat
WeChat